200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 114622-04-7
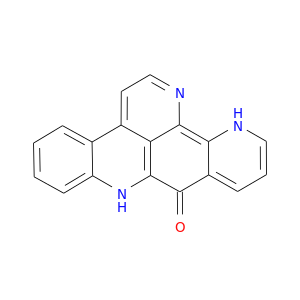
114622-04-7 | 9H-Quino[4,3,2-de][1,10]phenanthrolin-9-one
CAS No: 114622-04-7 Catalog No: AG000EUP MDL No:
Product Description
Catalog Number:
AG000EUP
Chemical Name:
9H-Quino[4,3,2-de][1,10]phenanthrolin-9-one
CAS Number:
114622-04-7
Molecular Formula:
C18H11N3O
Molecular Weight:
285.2994
IUPAC Name:
2,12,15-triazapentacyclo[11.7.1.03,8.09,21.014,19]henicosa-1,3,5,7,9(21),10,12,14(19),15,17-decaen-20-one
InChI:
InChI=1S/C18H9N3O/c22-18-12-5-3-8-19-15(12)16-14-11(7-9-20-16)10-4-1-2-6-13(10)21-17(14)18/h1-9H
InChI Key:
BTAIBIXHXSXUFN-UHFFFAOYSA-N
SMILES:
O=C1c2cccnc2c2c3c1nc1ccccc1c3ccn2
UNII:
855096HP36
NSC Number:
675670
Properties
Complexity:
467
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
283.075g/mol
Formal Charge:
0
Heavy Atom Count:
22
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
283.29g/mol
Monoisotopic Mass:
283.075g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
55.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.8
Literature
| Title | Journal |
|---|---|
| Synthesis and antimycobacterial activity of analogues of the bioactive natural products sampangine and cleistopholine. | European journal of medicinal chemistry 20130901 |
| Total synthesis of ascididemin via anionic cascade ring closure. | Chemical communications (Cambridge, England) 20120918 |
| Synthesis and in vitro antitumor activity of ring C and D-substituted phenanthrolin-7-one derivatives, analogues of the marine pyridoacridine alkaloids ascididemin and meridine. | Bioorganic & medicinal chemistry 20040801 |
| Synthesis and in vitro antitumor activity of an isomer of the marine pyridoacridine alkaloid ascididemin and related compounds. | Bioorganic & medicinal chemistry 20031001 |
| Synthesis and in vitro antitumor activity of phenanthrolin-7-one derivatives, analogues of the marine pyridoacridine alkaloids ascididemin and meridine: structure-activity relationship. | Journal of medicinal chemistry 20030731 |
| Mechanism of ascididemin-induced cytotoxicity. | Chemical research in toxicology 20030201 |
| Synthesis and in vitro antitumor activity of novel ring D analogues of the marine pyridoacridine ascididemin: structure-activity relationship. | Journal of medicinal chemistry 20020815 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







