200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 113866-40-3
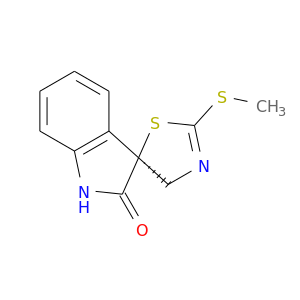
113866-40-3 | Spiro[3H-indole-3,5'(4'H)-thiazol]-2(1H)-one, 2'-(methylthio)-, (3S)-
CAS No: 113866-40-3 Catalog No: AG0009Y3 MDL No:
Product Description
Catalog Number:
AG0009Y3
Chemical Name:
Spiro[3H-indole-3,5'(4'H)-thiazol]-2(1H)-one, 2'-(methylthio)-, (3S)-
CAS Number:
113866-40-3
Molecular Formula:
C11H10N2OS2
Molecular Weight:
250.3399
IUPAC Name:
(3S)-2'-methylsulfanylspiro[1H-indole-3,5'-4H-1,3-thiazole]-2-one
InChI:
InChI=1S/C11H10N2OS2/c1-15-10-12-6-11(16-10)7-4-2-3-5-8(7)13-9(11)14/h2-5H,6H2,1H3,(H,13,14)/t11-/m1/s1
InChI Key:
FUHQSEOSBHASCH-LLVKDONJSA-N
SMILES:
CSC1=NC[C@]2(S1)C(=O)Nc1c2cccc1
Properties
Complexity:
358
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
250.023g/mol
Formal Charge:
0
Heavy Atom Count:
16
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
250.334g/mol
Monoisotopic Mass:
250.023g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
92.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.8
Literature
| Title | Journal |
|---|---|
| Catalytic enantioselective Henry reactions of isatins: application in the concise synthesis of (S)-(-)-spirobrassinin. | Chemistry (Weinheim an der Bergstrasse, Germany) 20110704 |
| Trypanosoma cruzi: antiproliferative effect of indole phytoalexins on intracellular amastigotes in vitro. | Experimental parasitology 20090501 |
| The phytopathogenic fungus Alternaria brassicicola: phytotoxin production and phytoalexin elicitation. | Phytochemistry 20090201 |
| Remarkable incorporation of the first sulfur containing indole derivative: another piece in the biosynthetic puzzle of crucifer phytoalexins. | Organic & biomolecular chemistry 20080107 |
| Determination of the enantiomeric purity of the phytoalexins spirobrassinins by 1H NMR using chiral solvation. | Bioorganic & medicinal chemistry letters 20041115 |
| Antiproliferative and cancer chemopreventive activity of phytoalexins: focus on indole phytoalexins from crucifers. | Neoplasma 20030101 |
| Synthesis, absolute configuration, and enantiomeric enrichment of a cruciferous oxindole phytoalexin, (S)-(-)-spirobrassinin, and its oxazoline analog. | The Journal of organic chemistry 20010601 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







