200,000+ products from a single source!
sales@angenechem.com
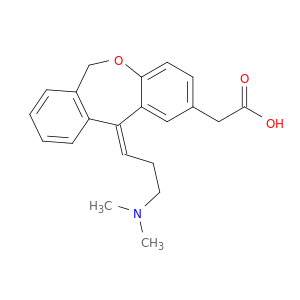
113806-05-6 | Dibenz[b,e]oxepin-2-acetic acid, 11-[3-(dimethylamino)propylidene]-6,11-dihydro-, (11Z)-
CAS No: 113806-05-6 Catalog No: AG0009QY MDL No:MFCD00865645
Product Description
Catalog Number:
AG0009QY
Chemical Name:
Dibenz[b,e]oxepin-2-acetic acid, 11-[3-(dimethylamino)propylidene]-6,11-dihydro-, (11Z)-
CAS Number:
113806-05-6
Molecular Formula:
C21H23NO3
Molecular Weight:
337.4122
MDL Number:
MFCD00865645
IUPAC Name:
2-[(11Z)-11-[3-(dimethylamino)propylidene]-6H-benzo[c][1]benzoxepin-2-yl]acetic acid
InChI:
InChI=1S/C21H23NO3/c1-22(2)11-5-8-18-17-7-4-3-6-16(17)14-25-20-10-9-15(12-19(18)20)13-21(23)24/h3-4,6-10,12H,5,11,13-14H2,1-2H3,(H,23,24)/b18-8-
InChI Key:
JBIMVDZLSHOPLA-LSCVHKIXSA-N
SMILES:
CN(CC/C=C/1\c2cc(ccc2OCc2c1cccc2)CC(=O)O)C
UNII:
D27V6190PM
Properties
Complexity:
488
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
1
Exact Mass:
337.168g/mol
Formal Charge:
0
Heavy Atom Count:
25
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
337.419g/mol
Monoisotopic Mass:
337.168g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
49.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.5
Literature
| Title | Journal |
|---|---|
| Identification of old drugs as potential inhibitors of HIV-1 integrase - human LEDGF/p75 interaction via molecular docking. | Journal of molecular modeling 20121201 |
| Advantages of histamine H4 receptor antagonist usage with H1 receptor antagonist for the treatment of murine allergic contact dermatitis. | Experimental dermatology 20120901 |
| [A 10-week safety and efficacy evaluation of olopatadine, 0.2% instilled twice-daily in patients with allergic conjunctivitis in Japan]. | Nippon Ganka Gakkai zasshi 20120901 |
| The antagonism of histamine H1 and H4 receptors ameliorates chronic allergic dermatitis via anti-pruritic and anti-inflammatory effects in NC/Nga mice. | Allergy 20120801 |
| Stereoselective syntheses of the antihistaminic drug olopatadine and its E-isomer. | The Journal of organic chemistry 20120720 |
| Loteprednol etabonate suspension 0.2% administered QID compared with olopatadine solution 0.1% administered BID in the treatment of seasonal allergic conjunctivitis: a multicenter, randomized, investigator-masked, parallel group study in Chinese patients. | Clinical therapeutics 20120601 |
| Effects of single therapeutic doses of promethazine, fexofenadine and olopatadine on psychomotor function and histamine-induced wheal- and flare-responses: a randomized double-blind, placebo-controlled study in healthy volunteers. | Archives of dermatological research 20120501 |
| The effect of antihistamines on seizures induced by increasing-current electroshocks: ketotifen, but not olopatadine, promotes the seizures in infant rats. | Biological & pharmaceutical bulletin 20120101 |
| Pharmacokinetic evaluation of olopatadine for the treatment of allergic rhinitis and conjunctivitis. | Expert opinion on drug metabolism & toxicology 20111201 |
| Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps). | PLoS computational biology 20111201 |
| Thermal angiooedema induced by hot water. | Acta dermato-venereologica 20110501 |
| Pre-seasonal treatment with topical olopatadine suppresses the clinical symptoms of seasonal allergic conjunctivitis. | American journal of ophthalmology 20110401 |
| A rapid and sensitive liquid chromatography-tandem mass spectrometry method for determination of olopatadine concentration in human plasma. | Journal of analytical toxicology 20110301 |
| Intrastromal corneal ring segments for bilateral keratoconus in an 11-year-old boy. | Journal of cataract and refractive surgery 20110101 |
| Comparison of effects of alcaftadine and olopatadine on conjunctival epithelium and eosinophil recruitment in a murine model of allergic conjunctivitis. | Drug design, development and therapy 20110101 |
| Olopatadine hydrochloride inhibits capsaicin-induced flare response in humans. | Pharmacology 20110101 |
| Two-week comparison study of olopatadine hydrochloride nasal spray 0.6% versus azelastine hydrochloride nasal spray 0.1% in patients with vasomotor rhinitis. | Allergy and asthma proceedings 20110101 |
| Comprehensive report of olopatadine 0.6% nasal spray as treatment for children with seasonal allergic rhinitis. | Allergy and asthma proceedings 20110101 |
| Olopatadine 0.6% nasal spray protects from vasomotor challenge in patients with severe vasomotor rhinitis. | American journal of rhinology & allergy 20110101 |
| Olopatadine suppresses the late phase reaction in parthenium dermatitis. | Indian journal of dermatology, venereology and leprology 20110101 |
| Stability-indicating high-performance column liquid chromatography and high-performance thin-layer chromatography methods for the determination of olopatadine hydrochloride in tablet dosage form. | Journal of AOAC International 20110101 |
| Dermatitis induced by estrogen and progesterone: dual positive results on the intradermal skin test. | Dermatitis : contact, atopic, occupational, drug 20110101 |
| Olopatadine hydrochloride in children: efficacy and safety for perennial allergic rhinitis. | Current medical research and opinion 20100701 |
| Comparison of olopatadine and fluorometholone in contact lens-induced papillary conjunctivitis. | Eye & contact lens 20100701 |
| Olopatadine nasal spray for the treatment of seasonal allergic rhinitis in patients aged 6 years and older. | Expert opinion on pharmacotherapy 20100601 |
| Olopatadine: a drug for allergic conjunctivitis targeting the mast cell. | Expert opinion on pharmacotherapy 20100401 |
| Olopatadine nasal spray for the treatment of allergic rhinitis. | Expert review of clinical immunology 20100301 |
| Histamine stimulates interleukin-6 production through histamine H1 receptors in human amnion cells. | Gynecologic and obstetric investigation 20100101 |
| Evaluation of olopatadine hydrochloride nasal spray, 0.6%, used in combination with an intranasal corticosteroid in seasonal allergic rhinitis. | Allergy and asthma proceedings 20100101 |
| Treatment of allergic conjunctivitis: results of a 1-month, single-masked randomized study. | European journal of ophthalmology 20100101 |
| [Toxicity of topical ocular anti-allergic agents on human corneal epithelial cells in vitro]. | [Zhonghua yan ke za zhi] Chinese journal of ophthalmology 20100101 |
| Comprehensive review of olopatadine: the molecule and its clinical entities. | Allergy and asthma proceedings 20100101 |
| Olopatadine hydrochloride improves dermatitis score and inhibits scratch behavior in NC/Nga mice. | International archives of allergy and immunology 20100101 |
| Synergetic effects of prednisolone and olopatadine on atopic dermatitis model of hairless mice. | Pharmacology 20100101 |
| Efficacy of oral olopatadine hydrochloride for the treatment of seasonal allergic rhinitis: A randomized, double-blind, placebo-controlled study. | Allergy and asthma proceedings 20100101 |
| Antihistaminic drug olopatadine downmodulates CCL17/TARC production by keratinocytes and Langerhans cells. | The Journal of dermatology 20091201 |
| Olopatadine hydrochloride inhibits scratching behavior induced by a proteinase-activated receptor 2 agonist in mice. | Journal of dermatological science 20091101 |
| Azelastine and olopatadine in the treatment of allergic rhinitis. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20091101 |
| Multiple action agents and the eye: do they really stabilize mast cells? | Current opinion in allergy and clinical immunology 20091001 |
| Efficacy of olopatadine HCI 0.1%, ketotifen fumarate 0.025%, epinastine HCI 0.05%, emedastine 0.05% and fluorometholone acetate 0.1% ophthalmic solutions for seasonal allergic conjunctivitis: a placebo-controlled environmental trial. | Acta ophthalmologica 20090801 |
| Cerebral histamine H1 receptor binding potential measured with PET under a test dose of olopatadine, an antihistamine, is reduced after repeated administration of olopatadine. | Journal of nuclear medicine : official publication, Society of Nuclear Medicine 20090601 |
| Severity scores, itch scores and plasma substance P levels in atopic dermatitis treated with standard topical therapy with oral olopatadine hydrochloride. | The Journal of dermatology 20090401 |
| Drug approvals: '08 in review. Olopatadine hydrochloride (Patanase) nasal spray. | The Nurse practitioner 20090201 |
| Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. | Journal of dermatological science 20090101 |
| Effects of olopatadine hydrochloride nasal spray 0.6% in the treatment of seasonal allergic rhinitis: a phase III, multicenter, randomized, double-blind, active- and placebo-controlled study in adolescents and adults. | Clinical therapeutics 20090101 |
| Is conjunctival allergen challenge a model of seasonal rhinoconjunctivitis? | Allergy and asthma proceedings 20090101 |
| Pharmacokinetics of orally administered single- and multiple-dose olopatadine in healthy Chinese subjects: an open-label study. | Clinical drug investigation 20090101 |
| Comparison of olopatadine 0.6% nasal spray versus fluticasone propionate 50 microg in the treatment of seasonal allergic rhinitis. | Allergy and asthma proceedings 20090101 |
| Evaluation of the cytotoxic effects of ophthalmic solutions containing benzalkonium chloride on corneal epithelium using an organotypic 3-D model. | BMC ophthalmology 20090101 |
| Evaluation of the efficacy and safety of olopatadine and fexofenadine compared with placebo in Japanese cedar pollinosis using an environmental exposure unit. | Journal of investigational allergology & clinical immunology 20090101 |
| Efficacy of repeated pretreatment with olopatadine hydrochloride on rhinitis induced by intranasal instillation of toluene-2,4-diisocyanate in rats. | Pharmacology 20090101 |
| Safety and efficacy of olopatadine hydrochloride nasal spray 0.6% in pediatric subjects with allergic rhinitis. | Allergy and asthma proceedings 20090101 |
| [Effectiveness of olopatadine therapy in seasonal allergic conjunctivitis]. | Oftalmologia (Bucharest, Romania : 1990) 20090101 |
| [Comparison of olopatadin and ketotifen in the treatment of allergic conjunctivitis]. | Revista medica del Instituto Mexicano del Seguro Social 20090101 |
| Comparison of the conjunctival toxicity of topical ocular antiallergic agents. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20081201 |
| Repeated pre-treatment with antihistamines suppresses [corrected] transcriptional up-regulations of histamine H(1) receptor and interleukin-4 genes in toluene-2,4-diisocyanate-sensitized rats. | Journal of pharmacological sciences 20081201 |
| Effects of olopatadine in limited scleroderma with peripheral eosinophils. | Geriatrics & gerontology international 20080901 |
| Evaluation of the antihistamine effects of olopatadine, cetirizine and fexofenadine during a 24 h period: a double-blind, randomized, crossover, placebo-controlled comparison in skin responses induced by histamine iontophoresis. | Archives of dermatological research 20080701 |
| The effects of the nasal antihistamines olopatadine and azelastine in nasal allergen provocation. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20080701 |
| Effect of olopatadine hydrochloride, an anti-histamine drug, on rhinitis induced by intranasal instillation of toluene-2,4-diisocyanate in rats. | International immunopharmacology 20080601 |
| Comparative efficacy of topical antihistamines in an animal model of early phase allergic conjunctivitis. | Experimental eye research 20080501 |
| Olopatadine 0.2% ophthalmic solution: the first ophthalmic antiallergy agent with once-daily dosing. | Expert opinion on drug metabolism & toxicology 20080401 |
| Analysis of disease-dependent sedative profiles of H(1)-antihistamines by large-scale surveillance using the visual analog scale. | Methods and findings in experimental and clinical pharmacology 20080401 |
| Progress in allergy signal research on mast cells: up-regulation of histamine signal-related gene expression in allergy model rats. | Journal of pharmacological sciences 20080301 |
| Evaluation of the effects of olopatadine ophthalmic solution, 0.2% on the ocular surface of patients with allergic conjunctivitis and dry eye. | Current medical research and opinion 20080201 |
| Olopatadine ameliorates rat experimental cutaneous inflammation by improving skin barrier function. | Pharmacology 20080101 |
| The noncompetitive antagonism of histamine H1 receptors expressed in Chinese hamster ovary cells by olopatadine hydrochloride: its potency and molecular mechanism. | Pharmacology 20080101 |
| P-glycoprotein limits the brain penetration of olopatadine hydrochloride, H1-receptor antagonist. | Drug metabolism and pharmacokinetics 20080101 |
| Enhancement of basophil apoptosis by olopatadine and theophylline. | Allergy and asthma proceedings 20080101 |
| A comparison of olopatadine 0.2% ophthalmic solution versus fluticasone furoate nasal spray for the treatment of allergic conjunctivitis. | Allergy and asthma proceedings 20080101 |
| Comparative study of sensory attributes of two antihistamine nasal sprays: olopatadine 0.6% and azelastine 0.1%. | Allergy and asthma proceedings 20080101 |
| Use of olopatadine ophthalmic solution and reactivity of histamine skin testing. | Allergy and asthma proceedings 20080101 |
| An assessment of the onset and duration of action of olopatadine nasal spray. | Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 20071201 |
| Efficacy of once-daily olopatadine 0.2% ophthalmic solution compared to twice-daily olopatadine 0.1% ophthalmic solution for the treatment of ocular itching induced by conjunctival allergen challenge. | Current eye research 20071201 |
| Ocular anti-allergic compounds selectively inhibit human mast cell cytokines in vitro and conjunctival cell infiltration in vivo. | Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 20071101 |
| [Pharmacological profile and clinical efficacy of olopatadine hydrochloride ophthalmic solution (Patanol 0.1% ophthalmic solution)]. | Nihon yakurigaku zasshi. Folia pharmacologica Japonica 20070901 |
| Duration of action of topical antiallergy drugs in a Guinea pig model of histamine-induced conjunctival vascular permeability. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20070801 |
| Safety and tolerability of olopatadine 0.2% in children and adolescents. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20070801 |
| The effect of H1 antagonists carebastine and olopatadine on histamine induced expression of CC chemokines in cultured human nasal epithelial cells. | Allergology international : official journal of the Japanese Society of Allergology 20070601 |
| Efficacy and comfort of olopatadine 0.2% versus epinastine 0.05% ophthalmic solution for treating itching and redness induced by conjunctival allergen challenge. | Current medical research and opinion 20070601 |
| Olopatadine hydrochloride accelerates the recovery of skin barrier function in mice. | The British journal of dermatology 20070501 |
| Effect of topical olopatadine and epinastine in the botulinum toxin B-induced mouse model of dry eye. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20070201 |
| Inhibition of cytokine-induced expression of T-cell cytokines by antihistamines. | Journal of investigational allergology & clinical immunology 20070101 |
| Efficacy of olopatadine ophthalmic solution 0.2% in reducing signs and symptoms of allergic conjunctivitis. | Allergy and asthma proceedings 20070101 |
| Chronic red eyes. Diagnosis: allergic conjunctivitis due to airborne allergen. | Comprehensive ophthalmology update 20070101 |
| Onset and duration of action of nasal sprays in seasonal allergic rhinitis patients: olopatadine hydrochloride versus mometasone furoate monohydrate. | Allergy and asthma proceedings 20070101 |
| Perception and quality of life associated with the use of olopatadine 0.2% (Pataday) in patients with active allergic conjunctivitis. | Advances in therapy 20070101 |
| Comprehensive report of the efficacy, safety, quality of life, and work impact of Olopatadine 0.6% and Olopatadine 0.4% treatment in patients with seasonal allergic rhinitis. | Allergy and asthma proceedings 20070101 |
| Olopatadine attenuates the enhancement of capsaicin-evoked substance P release by bradykinin from cultured dorsal root ganglion neurons. | European journal of pharmacology 20061215 |
| Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. | Eye & contact lens 20061201 |
| Neuronal conditions of spinal cord in dermatitis are improved by olopatadine. | European journal of pharmacology 20061010 |
| [Clinical effects of olopatadine hydrochloride on pruritus in skin diseases]. | Arerugi = [Allergy] 20061001 |
| Efficacy and response with olopatadine versus epinastine in ocular allergic symptoms: a post hoc analysis of data from a conjunctival allergen challenge study. | Clinical therapeutics 20061001 |
| [The use of 'Antigrippin-maximum' in the complex therapy of patients with acute respiratory diseases]. | Voenno-meditsinskii zhurnal 20061001 |
| [First Hungarian report of olopatadine eyedrop therapy in children and adults suffering from seasonal allergic conjunctivitis]. | Orvosi hetilap 20060910 |
| Health economic impact of olopatadine compared to branded and generic sodium cromoglycate in the treatment of seasonal allergic conjunctivitis in the UK. | Current medical research and opinion 20060901 |
| Allergic tears promote upregulation of eosinophil adhesion to conjunctival epithelial cells in an ex vivo model: inhibition with olopatadine treatment. | Investigative ophthalmology & visual science 20060801 |
| [Misleading advertisement on Opatanol]. | Nederlands tijdschrift voor geneeskunde 20060701 |
| Pharmacotherapy of allergic eye disease. | Expert opinion on pharmacotherapy 20060601 |
| Red, itchy eyes, giant papillae. | Advance for nurse practitioners 20060301 |
| Role of substance P in allergic nasal symptoms in rats. | European journal of pharmacology 20060217 |
| Comparison of the efficacy of olopatadine hydrochloride 0.1% ophthalmic solution and artificial tears in seasonal allergic conjunctivitis. | Acta ophthalmologica Scandinavica 20060201 |
| Histamine-induced vasodilation and vasoconstriction in the mesenteric resistance artery of the rat. | European journal of pharmacology 20060104 |
| Brain histamine H receptor occupancy of orally administered antihistamines measured by positron emission tomography with (11)C-doxepin in a placebo-controlled crossover study design in healthy subjects: a comparison of olopatadine and ketotifen. | British journal of clinical pharmacology 20060101 |
| Olopatadine suppresses the migration of THP-1 monocytes induced by S100A12 protein. | Mediators of inflammation 20060101 |
| Improved quality of life among seasonal allergic rhinitis patients treated with olopatadine HCl nasal spray 0.4% and olopatadine HCl nasal spray 0.6% compared with vehicle placebo. | Allergy and asthma proceedings 20060101 |
| [Comparative study between 0.025% ketotifen fumarate and 0.1% olopatadine hydrochloride in the treatment of vernal keratoconjunctivitis]. | Arquivos brasileiros de oftalmologia 20060101 |
| Effect of olopatadine and other histamine H1 receptor antagonists on the skin inflammation induced by repeated topical application of oxazolone in mice. | Pharmacology 20051201 |
| Safety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20051201 |
| The effects of olopatadine hydrochloride on the number of scratching induced by repeated application of oxazolone in mice. | European journal of pharmacology 20051107 |
| Safety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis to mountain cedar. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20051101 |
| Efficiency of olopatadine hydrochloride 0.1% in the treatment of vernal keratoconjunctivitis and goblet cell density. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20051001 |
| Effects of adjuvant therapy with 0.1% olopatadine hydrochloride ophthalmic solution on quality of life in patients with allergic rhinitis using systemic or nasal therapy. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20051001 |
| The various effects of four H1-antagonists on serum substance P levels in patients with atopic dermatitis. | The Journal of dermatology 20051001 |
| Mast cell stabilization and anti-histamine effects of olopatadine ophthalmic solution: a review of pre-clinical and clinical research. | Current medical research and opinion 20050901 |
| Comparison of the effects of ketotifen fumarate 0.025% and olopatadine HCl 0.1% ophthalmic solutions in seasonal allergic conjunctivities: a 30-day, randomized, double-masked, artificial tear substitute-controlled trial. | Clinical therapeutics 20050901 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Effects of a new formulation of olopatadine ophthalmic solution on nasal symptoms relative to placebo in two studies involving subjects with allergic conjunctivitis or rhinoconjunctivitis. | Current medical research and opinion 20050501 |
| A comparison of the clinical efficacy of pheniramine maleate/naphazoline hydrochloride ophthalmic solution and olopatadine hydrochloride ophthalmic solution in the conjunctival allergen challenge model. | Clinical therapeutics 20050501 |
| Effect of ketotifen fumarate, olopatadine, and levocabastine on ocular active anaphylaxis in the guinea pig and ocular immediate hypersensitivity in the albino rat. | Ocular immunology and inflammation 20050201 |
| [Examination of effectiveness of olopatadine hydrochloride in atopic dermatitis]. | Arerugi = [Allergy] 20050201 |
| Olopatadine hydrochloride suppresses the rebound phenomenon after discontinuation of treatment with a topical steroid in mice with chronic contact hypersensitivity. | Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 20050101 |
| Double-blind, crossover comparison of olopatadine and cetirizine versus placebo: suppressive effects on skin response to histamine iontophoresis. | The Journal of dermatology 20050101 |
| Effect of an antiallergic drug (Olopatadine hydrochloride) on TARC/CCL17 and MDC/CCL22 production by PBMCs from patients with atopic dermatitis. | Journal of dermatological science 20041201 |
| Effects of olopatadine hydrochloride, an antihistamine drug, on skin inflammation induced by repeated topical application of oxazolone in mice. | The British journal of dermatology 20041201 |
| Comparative efficacy of olopatadine 0.1% ophthalmic solution versus levocabastine 0.05% ophthalmic suspension using the conjunctival allergen challenge model. | Current medical research and opinion 20041201 |
| A review of olopatadine for the treatment of ocular allergy. | Expert opinion on pharmacotherapy 20040901 |
| Effects of bepotastine, cetirizine, fexofenadine, and olopatadine on histamine-induced wheal-and flare-response, sedation, and psychomotor performance. | Clinical and experimental dermatology 20040901 |
| Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. | Current medical research and opinion 20040801 |
| Clinical efficacy of olopatadine vs epinastine ophthalmic solution in the conjunctival allergen challenge model. | Current medical research and opinion 20040801 |
| Clinical efficacy of olopatadine hydrochloride ophthalmic solution 0.2% compared with placebo in patients with allergic conjunctivitis or rhinoconjunctivitis: a randomized, double-masked environmental study. | Clinical therapeutics 20040801 |
| Ocular allergy treatment comparisons: azelastine and olopatadine. | Current allergy and asthma reports 20040701 |
| A review of the use of olopatadine in allergic conjunctivitis. | International ophthalmology 20040501 |
| Differential regulation of IL-4 expression and degranulation by anti-allergic olopatadine in rat basophilic leukemia (RBL-2H3) cells. | Biochemical pharmacology 20040401 |
| [Olopatadine]. | Revue de l'infirmiere 20040401 |
| The promotion of eosinophil degranulation and adhesion to conjunctival epithelial cells by IgE-activated conjunctival mast cells. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20040101 |
| Preclinical and clinical antiallergic effect of olopatadine 0.2% solution 24 hours after topical ocular administration. | Allergy and asthma proceedings 20040101 |
| Properties of olopatadine hydrochloride, a new antiallergic/antihistaminic drug. | Arzneimittel-Forschung 20040101 |
| Comparative study of acute effects of single doses of fexofenadine, olopatadine, d-chlorpheniramine and placebo on psychomotor function in healthy volunteers. | Human psychopharmacology 20031201 |
| Interactions of olopatadine and selected antihistamines with model and natural membranes. | Ocular immunology and inflammation 20031201 |
| Double-masked, randomized, placebo-controlled clinical study of the mast cell-stabilizing effects of treatment with olopatadine in the conjunctival allergen challenge model in humans. | Clinical therapeutics 20031001 |
| Comparative study of 0.1% olopatadine hydrochloride and 0.5% ketorolac tromethamine in the treatment of seasonal allergic conjunctivitis. | Acta ophthalmologica Scandinavica 20030801 |
| Randomized, double-masked comparison of olopatadine ophthalmic solution, mometasone furoate monohydrate nasal spray, and fexofenadine hydrochloride tablets using the conjunctival and nasal allergen challenge models. | Clinical therapeutics 20030801 |
| [Opatanol]. | Ugeskrift for laeger 20030721 |
| One-visit, randomized, placebo-controlled, conjunctival allergen challenge study of scanning and imaging technology for objective quantification of eyelid swelling in the allergic reaction with contralateral use of olopatadine and artificial tears. | Clinical therapeutics 20030701 |
| Anti-allergic drug olopatadine suppresses murine contact hypersensitivity and downmodulates antigen-presenting ability of epidermal Langerhans cells. | Cellular immunology 20030701 |
| Evaluation of comfort using olopatadine hydrochloride 0.1% ophthalmic solution in the treatment of allergic conjunctivitis in contact lens wearers compared to placebo using the conjunctival allergen-challenge model. | Eye & contact lens 20030401 |
| [In vitro effects of antiallergic eyedrops on complement activation induced by particulate matter]. | Journal francais d'ophtalmologie 20030401 |
| Acute stress results in skin corticotropin-releasing hormone secretion, mast cell activation and vascular permeability, an effect mimicked by intradermal corticotropin-releasing hormone and inhibited by histamine-1 receptor antagonists. | International archives of allergy and immunology 20030301 |
| A randomized, double-blind, parallel-group comparison of olopatadine 0.1% ophthalmic solution versus placebo for controlling the signs and symptoms of seasonal allergic conjunctivitis and rhinoconjunctivitis. | Clinical therapeutics 20030301 |
| Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis: a real-world comparison of efficacy and ocular comfort. | Advances in therapy 20030101 |
| Effects of olopatadine, a new antiallergic agent, on human liver microsomal cytochrome P450 activities. | Drug metabolism and disposition: the biological fate of chemicals 20021201 |
| A comparison of the efficacy and tolerability of olopatadine hydrochloride 0.1% ophthalmic solution and cromolyn sodium 2% ophthalmic solution in seasonal allergic conjunctivitis. | Clinical therapeutics 20021001 |
| Compared topical ocular olopatadine 0.1% (Patanol) and loteprednol etabonate 0.2% (Alrex) in an allergen challenge model. | Clinical therapeutics 20020901 |
| Changes in tear function and the ocular surface after topical olopatadine treatment for allergic conjunctivitis: an open-label study. | Clinical therapeutics 20020801 |
| Comparison of the efficacy of combined fluticasone propionate and olopatadine versus combined fluticasone propionate and fexofenadine for the treatment of allergic rhinoconjunctivitis induced by conjunctival allergen challenge. | Clinical therapeutics 20020701 |
| Occupational allergy caused by Peruvian lily (Alstroemeria). | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20020601 |
| Comparison of the clinical efficacy and tolerability of olopatadine hydrochloride 0.1% ophthalmic solution and loteprednol etabonate 0.2% ophthalmic suspension in the conjunctival allergen challenge model. | Clinical therapeutics 20020601 |
| Azelastine is more potent than olopatadine n inhibiting interleukin-6 and tryptase release from human umbilical cord blood-derived cultured mast cells. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20020501 |
| Interaction of S100 proteins with the antiallergic drugs, olopatadine, amlexanox, and cromolyn: identification of putative drug binding sites on S100A1 protein. | Biochemical and biophysical research communications 20020412 |
| Pharmacological, pharmacokinetic and clinical properties of olopatadine hydrochloride, a new antiallergic drug. | Japanese journal of pharmacology 20020401 |
| Inhibitory effect of olopatadine on antigen-induced eosinophil infiltration and the LFA-1 and Mac-1 expression in eosinophils. | Japanese journal of pharmacology 20020401 |
| Azelastine's inhibition of histamine and tryptase release from human umbilical cord blood-derived cultured mast cells as well as rat skin mast cell-induced vascular permeability: comparison with olopatadine. | Allergy and asthma proceedings 20020101 |
| Olopatadine inhibits anti-immunoglobulin E-stimulated conjunctival mast cell upregulation of ICAM-1 expression on conjunctival epithelial cells. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20011101 |
| Effects of olopatadine hydrochloride on the cutaneous vascular hyperpermeability and the scratching behavior induced by poly-L-arginine in rats. | Japanese journal of pharmacology 20011001 |
| Mucus fishing syndrome: case report and new treatment option. | Optometry (St. Louis, Mo.) 20011001 |
| Evaluation of the efficacy of olopatadine hydrochloride 0.1% ophthalmic solution and azelastine hydrochloride 0.05% ophthalmic solution in the conjunctival allergen challenge model. | Clinical therapeutics 20010801 |
| [Pharmacological, pharmacokinetic and clinical properties of olopatadine hydrochloride' (olopatadine), an antiallergic drug]. | Nihon yakurigaku zasshi. Folia pharmacologica Japonica 20010701 |
| Olopatadine ophthalmic solution adjunctive to loratadine compared with loratadine alone in patients with active seasonal allergic conjunctivitis symptoms. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20010601 |
| [Effect of olopatadine hydrochloride, a novel antiallergic agent, on the QT interval in dogs]. | Nihon yakurigaku zasshi. Folia pharmacologica Japonica 20010601 |
| Inhibitory effect of olopatadine hydrochloride on the sneezing response induced by intranasal capsaicin challenge in guinea pigs. | Japanese journal of pharmacology 20010601 |
| Effects of olopatadine hydrochloride on the increase of histamine and peptide-leukotrienes concentrations in nasal lavage fluid following the antigen-antibody reaction in actively sensitized guinea pigs. | Japanese journal of pharmacology 20010401 |
| Comparative effects of topical ocular anti-allergy drugs on human conjunctival mast cells. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 19971201 |
| The in vitro and in vivo ocular pharmacology of olopatadine (AL-4943A), an effective anti-allergic/antihistaminic agent. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 19960101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







