200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1124-39-6
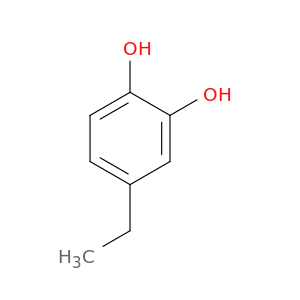
1124-39-6 | 4-Ethylbenzene-1,2-diol
CAS No: 1124-39-6 Catalog No: AG003LC1 MDL No:MFCD00015847
Product Description
Catalog Number:
AG003LC1
Chemical Name:
4-Ethylbenzene-1,2-diol
CAS Number:
1124-39-6
Molecular Formula:
C8H10O2
Molecular Weight:
138.1638
MDL Number:
MFCD00015847
IUPAC Name:
4-ethylbenzene-1,2-diol
InChI:
InChI=1S/C8H10O2/c1-2-6-3-4-7(9)8(10)5-6/h3-5,9-10H,2H2,1H3
InChI Key:
HFLGBNBLMBSXEM-UHFFFAOYSA-N
SMILES:
CCc1ccc(c(c1)O)O
EC Number:
214-397-5
UNII:
574JV8BYR2
Properties
Complexity:
103
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
138.068g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
138.166g/mol
Monoisotopic Mass:
138.068g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
40.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.5
Literature
| Title | Journal |
|---|---|
| Implications of Lactobacillus collinoides and Brettanomyces/Dekkera anomala in phenolic off-flavour defects of ciders. | International journal of food microbiology 20120201 |
| De-novo assembly and analysis of the heterozygous triploid genome of the wine spoilage yeast Dekkera bruxellensis AWRI1499. | PloS one 20120101 |
| Screening of representative cider yeasts and bacteria for volatile phenol-production ability. | Food microbiology 20111001 |
| Anti-influenza activity of marchantins, macrocyclic bisbibenzyls contained in liverworts. | PloS one 20110101 |
| The synthesis, structure and activity evaluation of pyrogallol and catechol derivatives as Helicobacter pylori urease inhibitors. | European journal of medicinal chemistry 20101101 |
| Anti-influenza activity of phenethylphenylphthalimide analogs derived from thalidomide. | Bioorganic & medicinal chemistry 20100715 |
| Determination of 4-ethylcatechol in wine by high-performance liquid chromatography-coulometric electrochemical array detection. | Analytica chimica acta 20080225 |
| Quantitative studies on the formation of phenol/2-furfurylthiol conjugates in coffee beverages toward the understanding of the molecular mechanisms of coffee aroma staling. | Journal of agricultural and food chemistry 20070516 |
| Synthesis and structure determination of covalent conjugates formed from the sulfury-roasty-smelling 2-furfurylthiol and di- or trihydroxybenzenes and their identification in coffee brew. | Journal of agricultural and food chemistry 20061227 |
| Quantitative precursor studies on di- and trihydroxybenzene formation during coffee roasting using 'in bean' model experiments and stable isotope dilution analysis. | Journal of agricultural and food chemistry 20061227 |
| Development of a stable isotope dilution analysis with liquid chromatography-tandem mass spectrometry detection for the quantitative analysis of di- and trihydroxybenzenes in foods and model systems. | Journal of agricultural and food chemistry 20060809 |
| Newbouldiosides A-C, phenylethanoid glycosides from the stem bark of Newbouldia laevis. | Phytochemistry 20060401 |
| Metabolic activation of carcinogenic ethylbenzene leads to oxidative DNA damage. | Chemico-biological interactions 20041207 |
Related Products
© 2019 Angene International Limited. All rights Reserved.