200,000+ products from a single source!
sales@angenechem.com
Home > Imidazoles > 104184-01-2
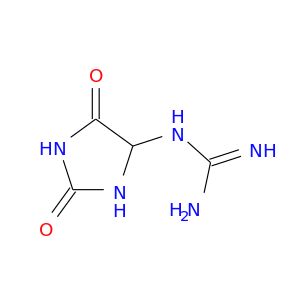
104184-01-2 | Guanidine, (2,5-dioxo-4-imidazolidinyl)-
CAS No: 104184-01-2 Catalog No: AG007EM4 MDL No:MFCD11040904
Product Description
Catalog Number:
AG007EM4
Chemical Name:
Guanidine, (2,5-dioxo-4-imidazolidinyl)-
CAS Number:
104184-01-2
Molecular Formula:
C4H7N5O2
Molecular Weight:
157.1307
MDL Number:
MFCD11040904
IUPAC Name:
2-(2,5-dioxoimidazolidin-4-yl)guanidine
InChI:
InChI=1S/C4H7N5O2/c5-3(6)7-1-2(10)9-4(11)8-1/h1H,(H4,5,6,7)(H2,8,9,10,11)
InChI Key:
AVOMHQCKYPXFNU-UHFFFAOYSA-N
SMILES:
NC(=N)NC1NC(=O)NC1=O
Properties
Complexity:
231
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
157.06g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
157.133g/mol
Monoisotopic Mass:
157.06g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
123A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-2.3
Literature
| Title | Journal |
|---|---|
| Structural context effects in the oxidation of 8-oxo-7,8-dihydro-2'-deoxyguanosine to hydantoin products: electrostatics, base stacking, and base pairing. | Journal of the American Chemical Society 20120912 |
| Thermodynamic consequences of the hyperoxidized guanine lesion guanidinohydantoin in duplex DNA. | Chemical research in toxicology 20120820 |
| Unzipping kinetics of duplex DNA containing oxidized lesions in an α-hemolysin nanopore. | Journal of the American Chemical Society 20120704 |
| Mutagenicity of secondary oxidation products of 8-oxo-7,8-dihydro-2'-deoxyguanosine 5'-triphosphate (8-hydroxy-2'- deoxyguanosine 5'-triphosphate). | Mutation research 20110901 |
| Substitution of Ala for Tyr567 in RB69 DNA polymerase allows dAMP and dGMP to be inserted opposite Guanidinohydantoin . | Biochemistry 20101005 |
| Crystal structure of a replicative DNA polymerase bound to the oxidized guanine lesion guanidinohydantoin. | Biochemistry 20100323 |
| Mutation versus repair: NEIL1 removal of hydantoin lesions in single-stranded, bulge, bubble, and duplex DNA contexts. | Biochemistry 20100302 |
| Influence of substrate complexity on the diastereoselective formation of spiroiminodihydantoin and guanidinohydantoin from chromate oxidation. | Chemical research in toxicology 20100215 |
| Plant and fungal Fpg homologs are formamidopyrimidine DNA glycosylases but not 8-oxoguanine DNA glycosylases. | DNA repair 20090501 |
| Mechanistic aspects of the formation of guanidinohydantoin from spiroiminodihydantoin under acidic conditions. | Chemical research in toxicology 20090316 |
| Calculation of pKa values of nucleobases and the guanine oxidation products guanidinohydantoin and spiroiminodihydantoin using density functional theory and a polarizable continuum model. | The journal of physical chemistry. B 20081225 |
| Superior removal of hydantoin lesions relative to other oxidized bases by the human DNA glycosylase hNEIL1. | Biochemistry 20080708 |
| An exploration of mechanisms for the transformation of 8-oxoguanine to guanidinohydantoin and spiroiminodihydantoin by density functional theory. | Journal of the American Chemical Society 20080416 |
| Unusual structural features of hydantoin lesions translate into efficient recognition by Escherichia coli Fpg. | Biochemistry 20070821 |
| Quantitation of four guanine oxidation products from reaction of DNA with varying doses of peroxynitrite. | Chemical research in toxicology 20051201 |
| Oxidised guanidinohydantoin (Ghox) and spiroiminodihydantoin (Sp) are major products of iron- and copper-mediated 8-oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanosine oxidation. | Molecular bioSystems 20051201 |
| Hydantoin derivative formation from oxidation of 7,8-dihydro-8-oxo-2'-deoxyguanosine (8-oxodG) and incorporation of 14C-labeled 8-oxodG into the DNA of human breast cancer cells. | Bioorganic & medicinal chemistry letters 20050801 |
| Recognition of the oxidized lesions spiroiminodihydantoin and guanidinohydantoin in DNA by the mammalian base excision repair glycosylases NEIL1 and NEIL2. | DNA repair 20050102 |
| Formation of 13C-, 15N-, and 18O-labeled guanidinohydantoin from guanosine oxidation with singlet oxygen. Implications for structure and mechanism. | Journal of the American Chemical Society 20031119 |
| Effect of the oxidized guanosine lesions spiroiminodihydantoin and guanidinohydantoin on proofreading by Escherichia coli DNA polymerase I (Klenow fragment) in different sequence contexts. | Biochemistry 20031111 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







