200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 10257-34-8
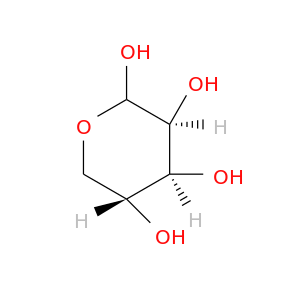
10257-34-8 | D-Lyxopyranose
CAS No: 10257-34-8 Catalog No: AG0007R7 MDL No:
Product Description
Catalog Number:
AG0007R7
Chemical Name:
D-Lyxopyranose
CAS Number:
10257-34-8
Molecular Formula:
C5H10O5
Molecular Weight:
150.1299
IUPAC Name:
(3S,4S,5R)-oxane-2,3,4,5-tetrol
InChI:
InChI=1S/C5H10O5/c6-2-1-10-5(9)4(8)3(2)7/h2-9H,1H2/t2-,3+,4+,5?/m1/s1
InChI Key:
SRBFZHDQGSBBOR-AGQMPKSLSA-N
SMILES:
O[C@@H]1COC([C@H]([C@H]1O)O)O
Properties
Complexity:
117
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
150.053g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
150.13g/mol
Monoisotopic Mass:
150.053g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
90.2A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-2.5
Literature
| Title | Journal |
|---|---|
| Characterization of a recombinant thermostable D-lyxose isomerase from Dictyoglomus turgidum that produces D-lyxose from D-xylulose. | Biotechnology letters 20120601 |
| Broadband dielectric spectroscopy and calorimetric investigations of D-lyxose. | Carbohydrate research 20111018 |
| Dibutylsilylene-pentose bis-chelates: on the glycoses' binding sites for strongly Lewis-acidic centres. | Carbohydrate research 20110927 |
| Synthesis of 4-amino-4,5-dideoxy-L-lyxofuranose derivatives and their evaluation as fucosidase inhibitors. | Carbohydrate research 20110715 |
| HPLC separation of all aldopentoses and aldohexoses on an anion-exchange stationary phase prepared from polystyrene-based copolymer and diamine: the effect of NaOH eluent concentration. | Molecules (Basel, Switzerland) 20110714 |
| On the stabilization of ribose by silicate minerals. | Astrobiology 20110301 |
| Characterization of a recombinant thermostable L: -rhamnose isomerase from Thermotoga maritima ATCC 43589 and its application in the production of L-lyxose and L-mannose. | Biotechnology letters 20101201 |
| Substrate specificity of a recombinant D-lyxose isomerase from Serratia proteamaculans that produces D-lyxose and D-mannose. | Letters in applied microbiology 20100901 |
| Substrate specificity of a recombinant D-lyxose isomerase from Providencia stuartii for monosaccharides. | Journal of bioscience and bioengineering 20100701 |
| Asymmetric histidine-catalyzed cross-aldol reactions of enolizable aldehydes: access to defined configured quaternary stereogenic centers. | Journal of the American Chemical Society 20091125 |
| Complexation behavior of mono- and disaccharides by the vinylbenzeneboronic acid-divinylbenzene copolymer resins packed in a high-performance liquid chromatographic column. | Journal of chromatography. A 20091030 |
| The crystal structure of a lyxose-bridged dimolybdate: a redetermination of the first monosaccharide-metal complex's structure. | Carbohydrate research 20090310 |
| Synthesis of 2-deoxy-D-arabino/lyxo-hexopyranosyl disaccharides. | Carbohydrate research 20080225 |
| Theoretical study on the factors controlling the stability of the borate complexes of ribose, arabinose, lyxose, and xylose. | Chemistry (Weinheim an der Bergstrasse, Germany) 20080101 |
| Crystal structure and solid-state 13C NMR analysis of N-p-nitrophenyl-alpha-D-ribopyranosylamine, N-p-nitrophenyl-alpha-D-xylopyranosylamine, and solid-state 13C NMR analysis of N-p-nitrophenyl-2,3,4-tri-O-acetyl-beta-D-lyxopyranosylamine and N-p-nitrophenyl-2,3,4-tri-O-acetyl-alpha-L-arabinopyranosylamine. | Carbohydrate research 20051212 |
| Genes encoding enzymes responsible for biosynthesis of L-lyxose and attachment of eurekanate during avilamycin biosynthesis. | Chemistry & biology 20051001 |
| Pentopyranosyl oligonucleotide systems. Part 11: Systems with shortened backbones: (D)-beta-ribopyranosyl-(4'-->3')- and (L)-alpha-lyxopyranosyl-(4'-->3')-oligonucleotides. | Bioorganic & medicinal chemistry 20010901 |
| A convenient route to higher sugars by two-carbon chain elongation using Wittig/dihydroxylation reactions. | The Journal of organic chemistry 20010629 |
| Design, synthesis, and antiviral activity of alpha-nucleosides: D- and L-isomers of lyxofuranosyl- and (5-deoxylyxofuranosyl)benzimidazoles. | Journal of medicinal chemistry 19980409 |
| L-lyxose metabolism employs the L-rhamnose pathway in mutant cells of Escherichia coli adapted to grow on L-lyxose. | Journal of bacteriology 19910801 |
Related Products
© 2019 Angene International Limited. All rights Reserved.