200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 102114-99-8
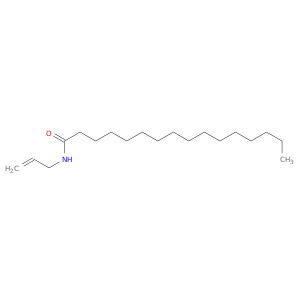
102114-99-8 | Hexadecanamide, N-2-propen-1-yl-
CAS No: 102114-99-8 Catalog No: AG0006O0 MDL No:
Product Description
Catalog Number:
AG0006O0
Chemical Name:
Hexadecanamide, N-2-propen-1-yl-
CAS Number:
102114-99-8
Molecular Formula:
C19H37NO
Molecular Weight:
295.5032
IUPAC Name:
N-prop-2-enylhexadecanamide
InChI:
InChI=1S/C19H37NO/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-19(21)20-18-4-2/h4H,2-3,5-18H2,1H3,(H,20,21)
InChI Key:
BZMVRGBTQHGPIW-UHFFFAOYSA-N
SMILES:
CCCCCCCCCCCCCCCC(=O)NCC=C
Properties
Complexity:
238
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
295.288g/mol
Formal Charge:
0
Heavy Atom Count:
21
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
295.511g/mol
Monoisotopic Mass:
295.288g/mol
Rotatable Bond Count:
16
Topological Polar Surface Area:
29.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
7.5
Literature
| Title | Journal |
|---|---|
| Randomized split-mouth study on postoperative effects of palmitoylethanolamide for impacted lower third molar surgery. | ISRN surgery 20110101 |
| Anxiety-like behaviour is attenuated by gabapentin, morphine and diazepam in a rodent model of HIV anti-retroviral-associated neuropathic pain. | Neuroscience letters 20081219 |
| Discovery and development of fatty acid amide hydrolase (FAAH) inhibitors. | Journal of medicinal chemistry 20081211 |
| The effect of the palmitoylethanolamide analogue, palmitoylallylamide (L-29) on pain behaviour in rodent models of neuropathy. | British journal of pharmacology 20070801 |
| Modifications of the ethanolamine head in N-palmitoylethanolamine: synthesis and evaluation of new agents interfering with the metabolism of anandamide. | Journal of medicinal chemistry 20030410 |
Related Products
© 2019 Angene International Limited. All rights Reserved.