200,000+ products from a single source!
sales@angenechem.com
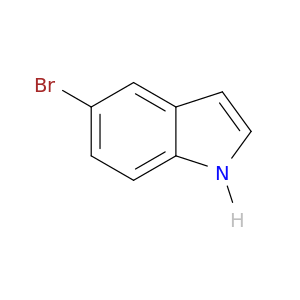
10075-50-0 | 1H-Indole, 5-bromo-
CAS No: 10075-50-0 Catalog No: AG0002SG MDL No:MFCD00005670
Product Description
Catalog Number:
AG0002SG
Chemical Name:
1H-Indole, 5-bromo-
CAS Number:
10075-50-0
Molecular Formula:
C8H6BrN
Molecular Weight:
196.0439
MDL Number:
MFCD00005670
IUPAC Name:
5-bromo-1H-indole
InChI:
InChI=1S/C8H6BrN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H
InChI Key:
VXWVFZFZYXOBTA-UHFFFAOYSA-N
SMILES:
Brc1ccc2c(c1)cc[nH]2
EC Number:
233-208-7
NSC Number:
75581
Properties
Complexity:
126
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
194.968g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
196.047g/mol
Monoisotopic Mass:
194.968g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
15.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3
Literature
| Title | Journal |
|---|---|
| Identification of novel CYP2A6 inhibitors by virtual screening. | Bioorganic & medicinal chemistry 20111201 |
| Biocatalysed halogenation of nucleobase analogues. | Biotechnology letters 20111001 |
| Evaluation of novel aminomethyl indole derivatives as Src kinase inhibitors and antioxidant agents. | Chemotherapy 20110101 |
| Synthesis and anticancer activity of 5-(3-indolyl)-1,3,4-thiadiazoles. | European journal of medicinal chemistry 20101001 |
| 1-[(E)-4-(5-Bromo-1H-indol-3-yl)-1-methyl-2,5,6,7-tetra-hydro-1H-azepin-2-yl-idene]propan-2-one. | Acta crystallographica. Section E, Structure reports online 20100701 |
| 1H NMR spectral studies on the polymerization mechanism of indole and its derivatives. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20060301 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Improved synthesis of aryltrialkoxysilanes via treatment of aryl Grignard or lithium reagents with tetraalkyl orthosilicates. | The Journal of organic chemistry 20041126 |
| Generation of new protein kinase inhibitors utilizing cytochrome p450 mutant enzymes for indigoid synthesis. | Journal of medicinal chemistry 20040603 |
| Hydroxylation of indole by laboratory-evolved 2-hydroxybiphenyl 3-monooxygenase. | The Journal of biological chemistry 20020913 |
Related Products
© 2019 Angene International Limited. All rights Reserved.