200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1002-26-2
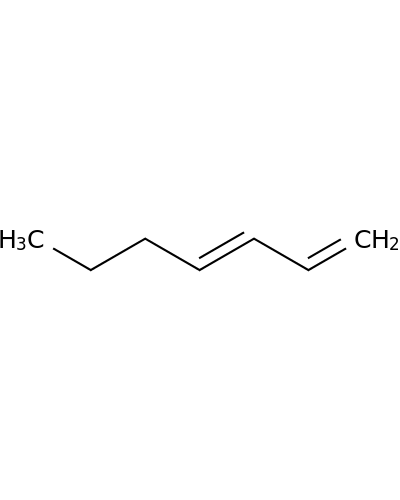
1002-26-2 | 1,3-Heptadiene
CAS No: 1002-26-2 Catalog No: AG00017N MDL No:
Product Description
Catalog Number:
AG00017N
Chemical Name:
1,3-Heptadiene
CAS Number:
1002-26-2
Molecular Formula:
C7H12
Molecular Weight:
96.1702
IUPAC Name:
hepta-1,3-diene
InChI:
InChI=1S/C7H12/c1-3-5-7-6-4-2/h3,5,7H,1,4,6H2,2H3
InChI Key:
OGQVROWWFUXRST-UHFFFAOYSA-N
SMILES:
CCCC=CC=C
Properties
Complexity:
60.4
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
96.094g/mol
Formal Charge:
0
Heavy Atom Count:
7
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
96.173g/mol
Monoisotopic Mass:
96.094g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
0A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
1
XLogP3:
3.1
Literature
| Title | Journal |
|---|---|
| Scavenging mechanism of curcumin toward the hydroxyl radical: a theoretical study of reactions producing ferulic acid and vanillin. | The journal of physical chemistry. A 20111215 |
| Bisdemethylcurcumin and structurally related hispolon analogues of curcumin exhibit enhanced prooxidant, anti-proliferative and anti-inflammatory activities in vitro. | Biochemical pharmacology 20100601 |
| Verrucisidinol and verrucosidinol acetate, two pyrone-type polyketides isolated from a marine derived fungus, Penicillium aurantiogriseum. | Marine drugs 20100101 |
| Curcumin-the paradigm of a multi-target natural compound with applications in cancer prevention and treatment. | Toxins 20100101 |
| Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. | Biochemical pharmacology 20081201 |
| Synthesis and evaluation of estrogen receptor ligands with bridged oxabicyclic cores containing a diarylethylene motif: estrogen antagonists of unusual structure. | Journal of medicinal chemistry 20051117 |
| Combined NMR and quantum chemical studies on the interaction between trehalose and dienes relevant to the antioxidant function of trehalose. | The journal of physical chemistry. B 20050224 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







